Introduction
Relapsed acute lymphoblastic leukemia (ALL) is a major therapeutic challenge and novel treatment strategies are urgently sought. RAS-pathway activating mutations are found in 38% of relapsed pediatric B-cell precursor (BCP) ALL patients ( J Irving, Blood, 2014). Additional mutations that activate the RAS-signalling cascade (NF1, BRAF, IKZF2, IKZF3, JAK1) have also been found with varying frequencies in relapsed ALL. Selumetinib, a potent MEK1/2 inhibitor, works synergistically with dexamethasone in mouse models of RAS-pathway mutated ALL by enhanced upregulation of the proapoptotic protein, BIM ( EC Matheson, Haematologica, 2019).
Methods
We conducted an international, parallel cohort, dose-finding and expansion trial to evaluate the combination of selumetinib with dexamethasone in adult and pediatric patients with relapsed/refractory T- or BCP-ALL harbouring RAS-pathway activating mutations. Selumetinib was supplied free of charge by AstraZeneca. Details of in-/exclusion criteria, treatment and dosages are described by T. Menne, BMJ open, 2022. The trial was conducted according to the Declaration of Helsinki and after medical ethics committees of participating countries had approved the protocol. The trial was registered under NCT03705507 and ISRCTN92323261 and sponsored by the University of Birmingham.
Results
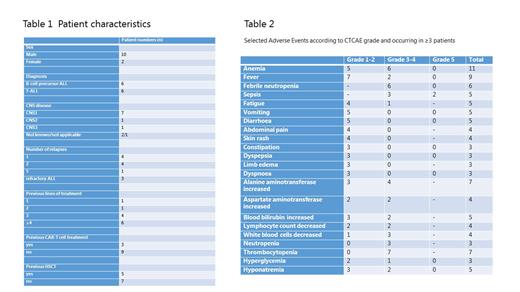
The trial opened to recruitment on 17-Apr-2018, the first patient was recruited on 18-May-2018. Twelve patients were included, 8 adults and 4 children. Six of the 8 adult patients had relapsed/refractory T-ALL, the remaining patients had BCP-ALL. Further patient characteristics are summarized in table 1.
Pharmacokinetic measurements demonstrated lower maximum concentrations for pediatric patients on dose level -1, and no significant effect of CYP3A4 induction was observed.
A total of 561 Adverse Events (AEs) were reported for 11/12 patients. For 1 patient who died on day 3 of treatment due to leukemia related events, no AEs were reported. 3/11 patients (27%) had AEs of a maximum grade 1-3, 5 patients (45%) had a maximum AE grade 4, and 3 patients (27%) suffered a grade 5 AE (2x sepsis, pneumonia). Further details of AEs are depicted in table 2.
Sixteen serious adverse events (SAEs) were observed in 9 patients. Of those, 11 were due to infection, and all 9 patients had an SAE involving infection, most frequently neutropenic fever or sepsis. Three SAEs were fatal: one concerned a child with sepsis who had stopped trial treatment 14 days previously. Two patients were adults: one became septic 2 days after being admitted for myopathy/weakness and collapsed shortly after starting antibiotics. The 2 nd patient was admitted with fever, diagnosed with pneumonia and died 5 days later of cardiac arrest. All fatal events occurred before the last of 3 urgent safety measures were implemented in September 2019, changing the continuous dexamethasone dosing to pulsed doses and mandating antimicrobial prophylaxis. The 5 non-infection SAEs were anaemia, constipation, myopathy/weakness, fatigue and heart failure.
Response data are available for 9/12 patients. Two patients died before reaching the 1 st bone marrow assessment after 28 days, and 1 patient stopped trial treatment after 11 days. Of the remaining 9 patients, 5 were non-responders (56%) and 4 (44%) had a complete remission (CR), or a CR with incomplete platelet recovery (3 adults, 1 child; response rate 44%). Of the 4 patients in CR, 2 adults had relapsed T-ALL and stayed on treatment for 3 to almost 5 months. Of the responding BCP-ALL patients, 1 patient died of pneumonia in cycle 2, and 1 pediatric patient progressed after cycle 2 then discontinued.
Conclusion
Recruitment to the trial was unexpectedly slow, due to competing new treatment options (commercial CAR-T cells, blinatumomab, inotuzumab), delays in opening international centres and recruitment stops due to COVID. Initially, the trial suffered from frequent SAEs concerning infections, which lead to a total of 3 urgent safety measures in 2018 and 2019, including lowering the dose of dexamethasone in the 2 nd half of cycle 1, mandating fluoroquinolone and co-trimoxazole prophylaxis and finally changing continuous dexamethasone to pulses. The response data with 44% of patients achieving a CR is promising and will need to be further evaluated in the context of targeted treatment approaches for RAS-altered leukemias, especially for relapsed T-ALL.
Disclosures
Menne:Kite/Gilead, Amgen, Novartis, Pfizer, Celgene/BMS, Daiichi Sankyo, Atara, F. Hoffmann-La Roche Ltd, Janssen, BMS, CTI BioPharma, Blueprint Medicines, Sanofi-Aventis, Spark Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months, Honoraria; Janssen, AstraZeneca, Novartis: Research Funding; Kite/Gilead, Takeda, Janssen, F. Hoffmann-La Roche Ltd, Servier, Novartis, Celgene/BMS, Pfizer, Incyte: Speakers Bureau; Amgen, Jazz, Pfizer, Bayer, Kyowa Kirin, Celgene/BMS, Kite/Gilead, Janssen, Takeda: Other: Travel grants; Kite/Gilead, Amgen, Novartis, Pfizer, Celgene/BMS, Daiichi Sankyo, Atara, Roche, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Fielding:Amgen, Incyte, Pfizer, Novartis: Consultancy. Morley:Abbvie: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Takeda: Honoraria. Kearns:AstraZeneca: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal